The Elements of Group IIIA - Group 3 elements in Periodic Table
The Elements of Group IIIA
Electronic Configuration of Group IIIA Elements
|
Element |
symbol |
electron configuration |
|
Boron |
B |
[He]2s2 2p1 |
|
Aluminium |
Al |
[Ne]3s22p1 |
|
Gallium |
Ga |
[Ar]4s2 2p1 |
|
Indium |
In |
[Kr]5s2 2p1 |
|
Thalium |
Tl |
[Xe]6s2 2p1 |
§ In passing from boron to thallium we encounter a change from semi metallic to metallic properties, from acidic to amphoteric to basic oxides, and from halides in which the bonding is distinctly covalent to halides in which bonding is nearly ionic.
Abundance:
§ The principal natural source of boron is the deposits of borax, Na2B4O.10H2O,
and recovery of the pure element from this compound is difficult.
§ On method that is used is the conversion of borax to the oxide B2O3,
by reduction with magnesium.
§ Aluminum is the most abundant metal in the earth’s crust and is
recovered pure and in quantity from its oxide by electrolytic reduction.
§ In contrast, gallium indium, and thallium are quite rare.
Properties of group IIIA
The trends in hardness, boiling temperature, and
Boron and Boron Compounds:
§ Although boron is formally in the +3 oxidation state in its oxide
and in halides, there no chemistry associated with a free B+3 ion.
§ The energy required to remove three electrons from the boron atom is
very large; moreover, the ion once formed would be extremely small and has
large polarizing power. So, boron form covalent compounds (tend to share
electrons)
Boron Halides:
The bond in Boron-Halide is covalent bond, and the boiling points of the boron halides increase as the atomic number of the halogen increases.
All the boron halides act as electron acceptors, as for example.
In these reactions BF3 accepts a pair of electrons donated by NH3 or F-. Thus, BF3 and the other boron halides are Lewis acids.
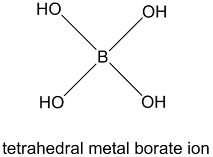
Boron Oxides:
In this reaction boron accept electrons, and act as Lewis acid and accepts electrons from OH-. In this case the hybridization of B is converted from Sp2 to Sp3.
Boranes:
When diborane is heated to temperatures between 100°C and 250oC, it converts to a number of other boranes.








No comments