What is photoelectric effect?
What is photoelectric effect?
Photoelectric Effect Definition
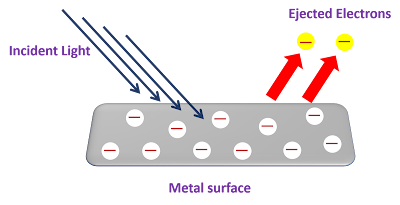
- In photoelectric effect the electrons are emitted instantaneously from clean metal surface in vacuum when a beam of light falls on it.
Characterization of photoelectric effect:
- Electrons are emitted instantaneously from clean metal when irradiated with radiation with frequency equal to or greater than minimum value called threshold frequency.
- The kinetic energy of the emitted electrons depends upon the frequency of the radiation and does not depend on its intensity, kinetic energy increase linearly with increase in frequency.
- The number of electrons emitted is proportional to the intensity of the incident light.
Work function:
- It is the energy supplied to electron before it can be removed from metal and it is equal to the potential energy of electron.
- If the emitted electron has kinetic energy, then it’s the total energy supplied by radiation will equal to the sum of its potential and kinetic energy.Failure of classical theory in explanation of photoelectric effect:
The classical theory failed to explain the following characteristics of photoelectric effect:
- The instantaneous emission of electrons.
- The existence of threshold frequency.
- The dependence of kinetic energy of emitted electron on frequency of light.
Why Classical theory failed to explain the photoelectric effect:
This because in classical theory the energy of light depend on its intensity so, a continuous exposure of metal with light causes electron to gain more and more energy till its energy become sufficient to its ionization, so, there must be time interval between the exposure and emission of electron, this time interval decrease by increasing the light intensity and all of this does not happen.
Einstein Explanation of photoelectric effect:
- Einstein stated that light itself is considered to be made of discrete particles called photons similarly as Max Planck assumption.
- Each photon carries energy equal to (h) and this energy transfer to electron when photon collide with the electrons of the metal.
- If the frequency of the incident radiation is smaller than “threshold frequency” the electron will not ionize.
- From equation (1), It is obvious that kinetic energy of electron depends on frequency of the incident radiation not on the intensity
- The intensity of radiation will have effect only on the number of electrons emitted
- From equation (1) and (2) we find that photoelectric effect equation is:












No comments